Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure
$ 8.99 · 4.8 (515) · In stock


As the pressure approaching zero i.e., very low pressure, the curves plotted between compressibility factor Z and P n mole of gases have the following characteristics.I. The intercept on the y-axis leads

PV Compressibility factor Z= nRT is plotted against pressure : N. Ideal gas What is the correct order of liquefiability of the gases shown in the above graph? H
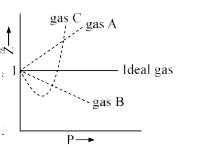
Gas C is a real gas and we can find 'a' and 'b' if intersection data i

Compressibility factorZPVnRTis plotted againstpressure What is the correct order of liquefiability of the gases shown in the above graph

Deviation Of Real Gas From Ideal Gas Behavior
Why the graph of a pressure against volume of a fixed amount of a gas a curve and not a straight line? - Quora

Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure
1.5 Real Gases and the Virial Equation - Mail
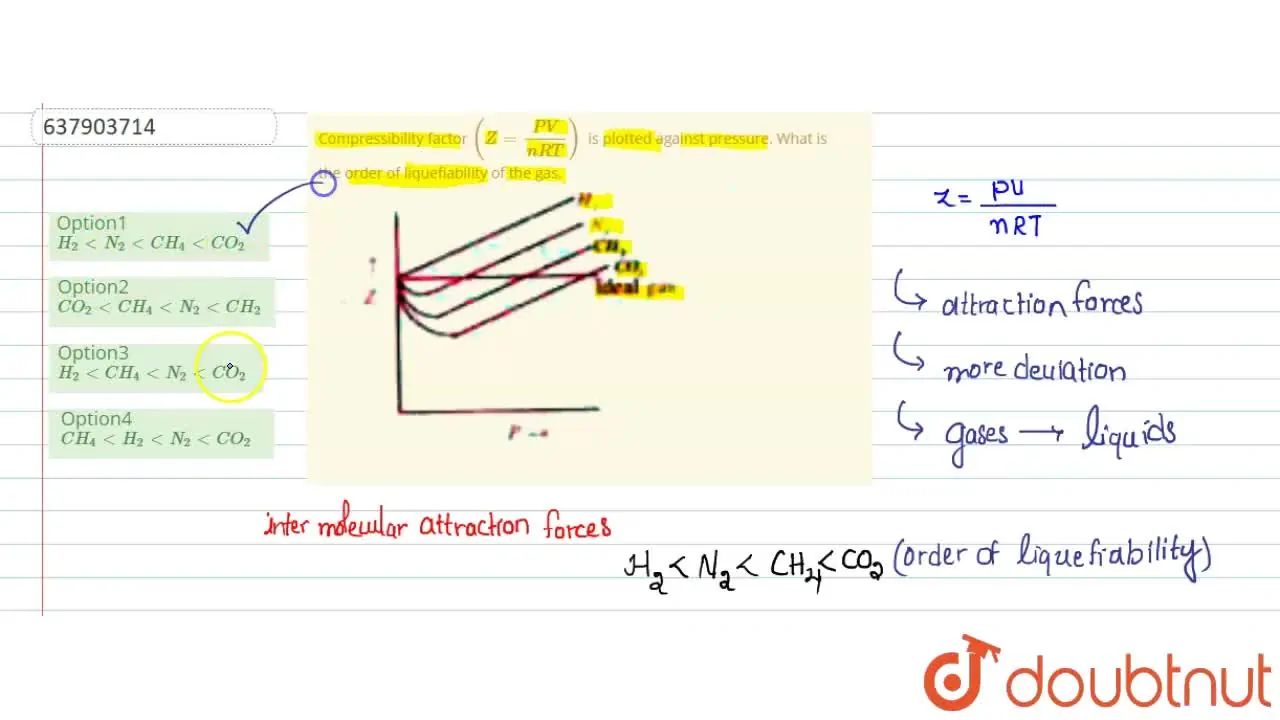
Telugu] Compressibility factor (Z = (PV)/(nRT)) is plotted against p
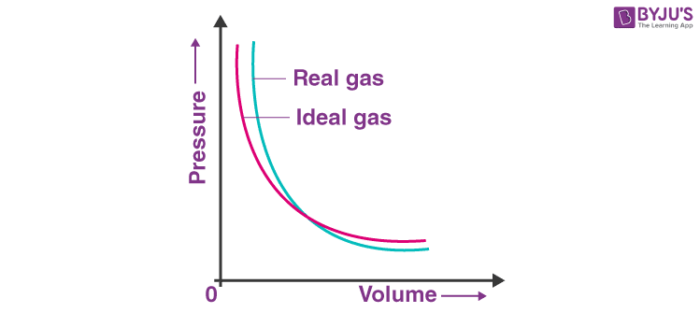
Deviation Of Real Gas From Ideal Gas Behavior

gas laws - Does the amount of a gas increase with pressure? - Chemistry Stack Exchange
Essential Pharma Documents: 1205: Properties of Gases

Is z (compressibility factor) vs P (pressure) graph drawn by changing volume? If it is why it isn't drawn by changing mole - Quora